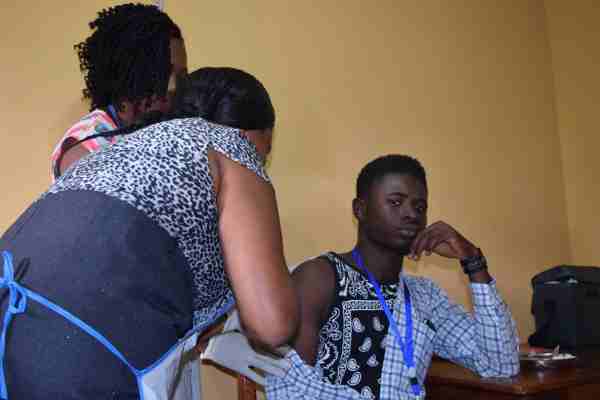Clinical Trial of Ebola Vaccine Regimen in Sierra Leone
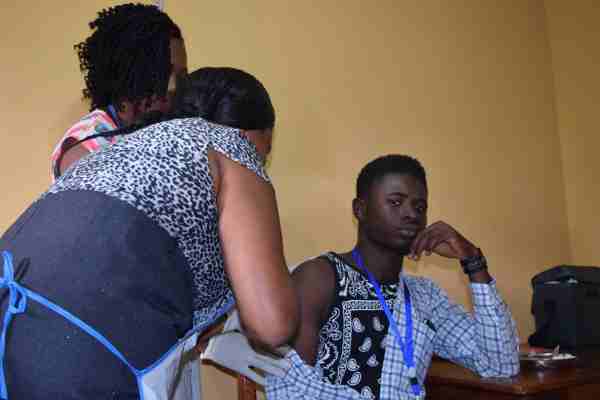
Johnson & Johnson (NYSE: JNJ) announced Friday the start of a safety and immunogenicity clinical trial in Sierra Leone of a preventive Ebola vaccine regimen in development at its Janssen Pharmaceutical Companies.
Trial recruitment is underway, and the first volunteers have received their initial vaccine dose. This is stated to be the first study conducted of Janssen’s Ebola prime-boost vaccine regimen in a West African country affected by the recent Ebola epidemic.
The new study, EBOVAC-Salone, will take place in Sierra Leone’s Kambia district, where some of the country’s most recent Ebola cases have been reported.
[ Panasonic Solar Lanterns for Ebola Affected Regions ]
The regimen being tested uses a combination of two vaccine components based on AdVac technology from Crucell Holland B.V., one of the Janssen Pharmaceutical Companies, and MVA-BN technology from Bavarian Nordic.
Volunteers in the study will first be given the AdVac dose to prime their immune system, and then the MVA-BN dose two months later to boost their immune response, with the goal of potentially strengthening and optimizing the duration of the immunity.